A Note to Readers
When I researched Charles Darwin for my book, Mightier Than the Sword, I was surprised to learn he frequently skipped school. But Darwin didn’t just mess around–(okay, he did a little of that)–he used his time away from school to experiment with chemistry. In fact, he got so good at making laughing gas that his classmates began calling him “Gas.”
When I heard about Sue Berk Koch’s book, Chemical Reactions–I couldn’t wait to feature it on the blog! How much fun is this? Plus, how else are you going to celebrate Periodic Table Day!
Happy Reading,
Rochelle
Teaching Chemical Reactions
Celebrate Periodic Table Day
by Making a Molecule
There are many national celebration days! Some are silly, such as National Walk Around Things Day or National Lumpy Rug Day. But National Periodic Table Day on February 7 is right up there with National Eat Ice Cream for Breakfast Day!
Kidding aside, the periodic table is one of chemistry’s most important breakthroughs. And it’s no coincidence that Demetri Mendeleev’s birthday — the Russian chemist credited with devising the periodic table, is February 6. That’s the day before National Periodic Table Day. Unfortunately, February 6 had already been established as National Lame Duck Day!
THE PERIODIC TABLE
The periodic table not only organizes the elements, but it provides an amazing, visual chart, chock full of information about each element and how they relate to each other. It is a systematic arrangement of the known and created elements in order of increasing atomic number, designed so that elements with similar properties together are grouped together. That’s a lot to accomplish in one chart! How did this happen?
THE HISTORY OF THE PERIODIC TABLE
During Mendeleev’s time (1834–1907), many elements were being discovered. Scientists were having difficulty keeping the elements organized.
Mendeleev thought to group elements together that acted alike. He created a grid of the elements, but to make it work, he had to leave gaps. This was a stroke of genius! He realized that the gaps represented elements that had not yet been discovered. His predictions were correct! Element 101, Mendelevium, is named after him.
To put this brilliance in perspective, Mendeleev compiled his periodic table almost 30 years before J.J. Thomson discovered the electron, close to 45 years before Ernest Rutherford found the nucleus of an atom and over 50 years before scientists determined that the proton and neutron made up the nucleus of the atom!
Every element on the periodic table is made up of only one type of atom.
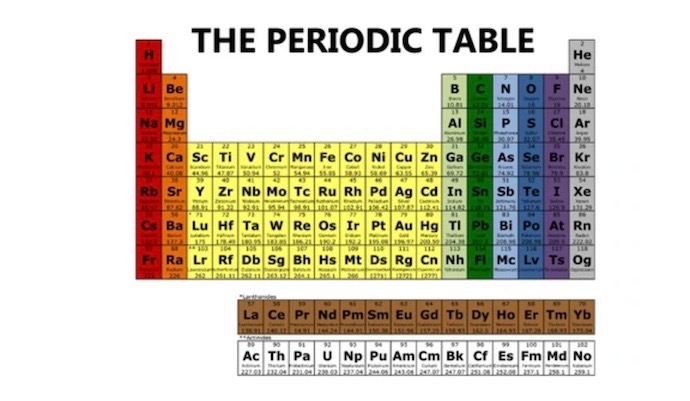
ALPHABET FOR CHEMISTRY
The periodic table can be thought of as a kind of alphabet for chemistry. The letters in the alphabet combine to make all the English words we use. The 118 elements on the periodic table can combine in different ways to make everything on Earth! The everything we’re talking about is matter. Matter is anything that takes up space and has mass. Matter is made up of atoms. Atoms combine to form molecules.
What about combining two elements from the periodic table to make the most abundant compound on Earth?
STEM ACTIVITY: MAKE YOUR OWN MOLECULE
To make a molecule, we’ll need a few atoms. As we know, atoms are the building blocks of all matter, including elements. When a group of different atoms bond together, they form a new substance. What is the most abundant compound on our planet? Water! Water is made up of two different elements on the periodic table, oxygen and hydrogen.
To begin we’ll need supplies.
SUPPLIES
* Toothpick
* 1 grape
* 2 blueberries
DIRECTIONS
- Break your toothpick in half and press one half into the grape, leaving part of it showing.
- Attach a blueberry to the other side of the toothpick.
- Put the other toothpick half in the grape. Arrange the toothpicks the way a cat’s ears would be.
- Put the other blueberry on that toothpick. You’ve made an edible model of a water molecule! The toothpicks are holding your fruit together the way a bond holds atoms. Bonds act like magnets. The bonds hold the hydrogen and oxygen atoms together, forming a molecule of water!
- You can eat your water molecule model but be careful not to eat the toothpick!
MORE LEARNING ABOUT CHEMISTRY AND CHEMICAL REACTIONS
 You can find chemistry everywhere! Especially in my book. In Chemical Reactions! With 25 Science Projects for Kids, readers ages 7 to 12 learn about the atoms and molecules that make up everything in our world and what happens when different atoms and molecules come in contact with each other.
You can find chemistry everywhere! Especially in my book. In Chemical Reactions! With 25 Science Projects for Kids, readers ages 7 to 12 learn about the atoms and molecules that make up everything in our world and what happens when different atoms and molecules come in contact with each other.
Explore chemical reactions through 25 hands-on, STEM activities, fascinating facts, essential questions, links to online resources, and even jokes that help support deeper learning!
If you’d like a pdf study guide of this activity and one more that shows how powerful releasing compressed gas can be, along with a before and after list of essential questions, you can find me on my website. https://susanberkkoch.com/blog Please sign up for my newsletter. We can help kids make sense of science together!
 About the author. Sue is an award-winning author of dozens of articles for children. Her work has been in magazines such as Highlights, Muse, Odyssey, Boys Quest, and Ask. Her book Chemical Reactions is available from Nomad Press. She has a B.S. in biology and chemistry, and her doctorate from Marquette University. She lives in Wisconsin with her husband, three boys, and a menagerie of pets. She makes sense of science on her blog here: https://susanberkkoch.com/blog
About the author. Sue is an award-winning author of dozens of articles for children. Her work has been in magazines such as Highlights, Muse, Odyssey, Boys Quest, and Ask. Her book Chemical Reactions is available from Nomad Press. She has a B.S. in biology and chemistry, and her doctorate from Marquette University. She lives in Wisconsin with her husband, three boys, and a menagerie of pets. She makes sense of science on her blog here: https://susanberkkoch.com/blog





